 |
Name: | Valeria Dantignana | |
| eMail: | valeria.dantignana@udg.edu | ||
| Institution: | Universitat de Girona | ||
| Start Date: | 1/5/2016 | Return | |
| Supervisors: | Prof. Dr. Miquel Costas | ||
| Dr. Anna Company | |||
Non-noble metal catalysts for light alkane oxidations
|
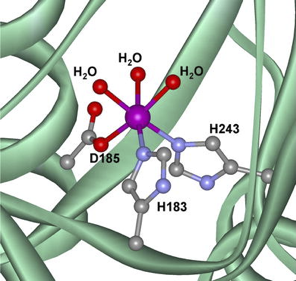
The oxidation of C-H bonds is of interest both in the chemical industry and in the biological processes.[1] However, the inertness of these bonds makes the development of systems able to perform this transformation specially challenging if the use of toxic reagents or harsh reaction conditions is avoided. In nature these transformations are efficiently catalyzed by metallo-enzymes, such as oxygenases (Figure 1a).[2,3] These proteins catalyze the insertion of one or two oxygen atoms in an organic substrate, using molecular oxygen or its reduced forms as oxidant, under mild reaction conditions. Given the high oxidative activity of oxygenases, which are both selective and highly efficient, and also the eco-compatibility of these enzymatic processes, a bio-inspired approach has been followed in the last three decades with the aim of mimicking the active site of these natural catalysts to develop artificial complexes in an attempt to obtain equally active catalytic systems.[4,5]
In particular, it has been observed that tripodal tetradentate ligands that coordinate the iron leaving the metal with two labile sites in a relative cis configuration give rise to especially efficient catalysts (Figure 1b), which act via a metal-oxo based pathway.[6] Considering the successful results obtained in C-H hydroxylation and C-C bond epoxidation and dihydroxylation reactions by using catalysts containing this ligand scaffold, in this project these complexes will be employed in the functionalization of strong C-H bonds, such as alkylic C-H bonds of non-activated alkanes.
 |
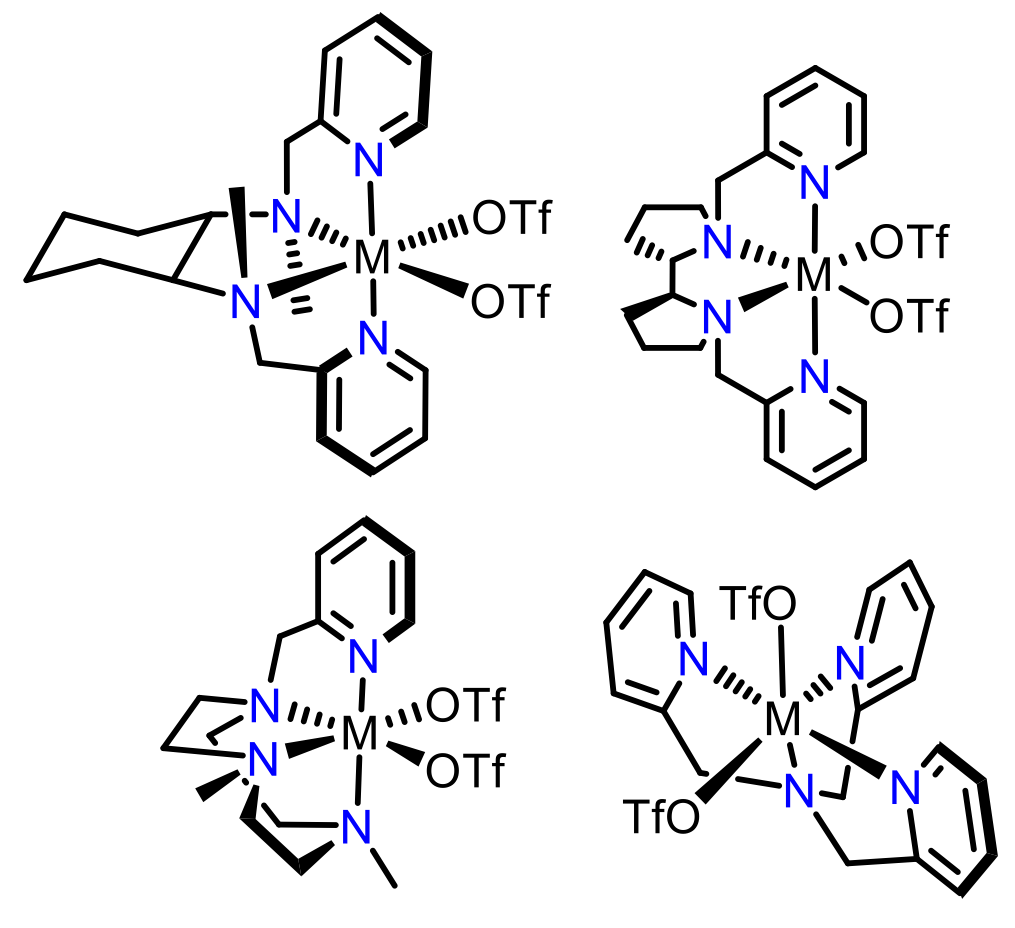 |
| Figure 1a: Active site Rieske oxygenase | Figure 1b: Bio-inspired catalysts |
References:
[1] F. Roudesly, J. Oble, G. Poli, Journal of Molecular Catalysis A: Chemical, 2017, 275.
[2] P.R. Ortiz de Montellano, Chem. Rev., 2010, 932.
[3] D.J. Ferraro, L. Gakhar, S. Ramaswamy, Biochemical and Biophysical Research Communications, 2005, 338.
[4] Z. Codola, J. Lloret-Fillol, M. Costas, Progress in Inorganic Chemistry 2014, 59, 447.
[5] E.P. Talsi, K.P. Bryliakov, Coord. Chem. Rev., 2012, 256, 1418. [6] W.N Oloo, L. Que Jr., Acc. Chem. Res., 2015, 48, 2612.