 |
Name: | Marco Cianfanelli | |
| eMail: | marco.cianfanelli89@gmail.com | ||
| Institution: | Universitat de Girona (UdG) | ||
| Start Date: | 1/5/2016 | Return | |
| Supervisors: | Prof. Dr. Miquel Costas | ||
Stereoselective C-H Oxidations with Fe and Mn Complexes
|
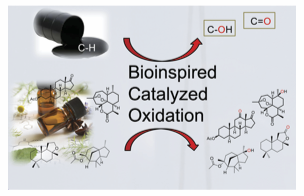 Catalytic C-H oxidation represents a powerful method to transform large available hydrocarbons or complex natural products in valuable oxygenated building blocks. However, due the inert nature of C-H bonds and their ubiquitous presence in organic molecules, an efficient and selective oxidation protocol still remains a challenge in modern organic chemistry.[1] Traditional reagents tend to be less selective, generally very toxic and require drastic reaction conditions. Contrarily, enzymatic oxidations are based on earth-abundant and non-toxic metals, mild reaction conditions and O2 or H2O2 as terminal oxidant. Therefore, an attractive strategy towards novel synthetic catalysts is the bioinspired ligand design that enables the mimicry of the first coordination sphere of non-heme metalloenzymes. In this regard, aminopyridine-based Fe and Mn coordination complexes (Figure 1) are emerging as promising candidates to reproduce the enzyme’s ability to efficiently activate O-O bond, generating powerful high-valent metal oxidant species in presence of carboxylic acids. [2]
Catalytic C-H oxidation represents a powerful method to transform large available hydrocarbons or complex natural products in valuable oxygenated building blocks. However, due the inert nature of C-H bonds and their ubiquitous presence in organic molecules, an efficient and selective oxidation protocol still remains a challenge in modern organic chemistry.[1] Traditional reagents tend to be less selective, generally very toxic and require drastic reaction conditions. Contrarily, enzymatic oxidations are based on earth-abundant and non-toxic metals, mild reaction conditions and O2 or H2O2 as terminal oxidant. Therefore, an attractive strategy towards novel synthetic catalysts is the bioinspired ligand design that enables the mimicry of the first coordination sphere of non-heme metalloenzymes. In this regard, aminopyridine-based Fe and Mn coordination complexes (Figure 1) are emerging as promising candidates to reproduce the enzyme’s ability to efficiently activate O-O bond, generating powerful high-valent metal oxidant species in presence of carboxylic acids. [2]
Ultimately, chemoselectivity, regioselectivity and stereoselectivity remain deeply dependent on the structural and electronic properties of these complexes. Thus, a better understanding of how the ligands influence the structural and reactivity properties of these metal species enables the optimization of these metal‐catalyzed oxidation reactions. Synthetic efforts are being carried out to develop ligands that tune the metal center´s reactivity for the selective oxidation of specific C-H bond in organic compounds of interest.
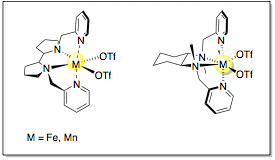 |
| Figure 1. General structures of linear aminopyridine-based Fe and Mn complexes |
References:
[1] T. Newhouse, P. S. Baran, Angew Chem. Int. Ed., 50 (2011), 3362.
[2] Z. Codolà, J. Lloret-Fillol, M. Costas in Prog. In Inorg. Chem. (2014), 447.