 |
Name: | Emily Monkcom | |
| eMail: | e.c.monkcom@uu.nl | ||
| Institution: | Utrecht University | ||
| Start Date: | 1/3/2016 | Return | |
| Supervisors: | Prof dr. Bert Klein Gebbink | ||
Iron-Mediated C-X Bond Coupling Through Direct C-H Activation
|
Mononuclear non-heme iron enzymes involved in oxidative transformations are capable not only of overcoming the kinetic barrier associated to the activation of molecular oxygen, but of achieving important metabolic or catabolic reactions with an exceptional level of substrate specificity, regio- and stereoselectivity. This project is specifically focused on the biomimicry of isopenicillin N synthase (IPNS), a unique metalloenzyme capable of activating O2 in order to achieve two heterocyclisation reactions during the conversion of its natural tripeptide substrate -(L--aminoadipoyl)-L-cysteinyl-D-valine (ACV) to isopenicillin N synthase (IPN).[1] During this transformation, O2 is reduced to two equivalents of water while two unactivated aliphatic C-H bonds are functionalised - an unprecedented feat in synthetic organic chemistry.
At its active site, IPNS bears the iconic 2-His-1-carboxylate (2H1C) facial triad, where the iron centre is facially capped by a tripodal arrangement of two His residues and a carboxylate group (from Asp/Glu) (A).[2] This serves as the founding architectural feature of the project’s bioinspired ligand design. The use of bulky 3,3-bis(1,5-dimethylbenzimidazole)-N-alkylpropanamide (B) ligands[3] as well as tris(4,4-dimethyloxazoline-2-yl)phenylborate (C) ligands[4] is being investigated as a suitable monoanionic, facially-capping tripodal ligand platform with which to synthesise monoligated iron complexes. Exploring the coordination chemistry of the resulting complexes with sulfur-rich substrates is hoped to provide faithful structural and functional models of IPNS and its coordination to ACV. Finally, we hope to push this chemistry further by achieving catalytic intramolecular cyclisation reactions in which C-H is activated and new C-heteroatom bonds are formed.
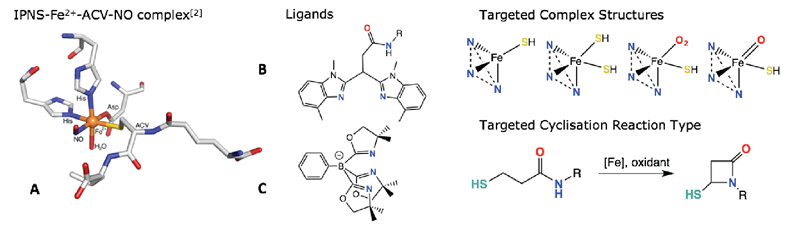
Figure 1. A) The substrate-bound active site of IPNS; B) the general structure for BMMeBIPAR ligands; C) the ToM ligand. The targeted complex structures and catalytic schemes are also shown.
[1] E. Tamanaha et al., J. Am. Chem. Soc. 138, 8862-8874 (2016)
[2] P. L. Roach et al., Nature 387, 827-830 (1997)
[3] E. Folkertsma et al., Eur. J. Inorg. Chem., 1319-1332 (2016)
[4] R. R. Reinig et al., Eur, J. Inorg. Chem., 2486-2494 (2016)