 |
Name: | Eduard Masferrer Rius | |
| eMail: | e.masferrerrius@uu.nl | ||
| Institution: | Utrecht University | ||
| Start Date: | 1/12/2016 | Return | |
| Supervisors: | Prof dr. Bert Klein Gebbink | ||
Direct aromatic oxygenation reactions catalyzed by nickel complexes supported by aminopyridine ligands
|
During last years, improvements have been made in the development of oxidation catalysis, such as the direct conversion of aryl or alkyl C-H bonds into the corresponding C-OH functionality. However, the selective oxidation of organic substrates, such as methane or benzene, belongs to one of the most challenging fields of contemporary chemical research, both in industrial and academic areas.
This research project is focused on the investigation of new homogeneous catalysts for the direct aromatic hydroxylation of benzene (and substituted benzenes) to phenol using different benign oxidants, such as H2O2 or O2. Worthy of note is that phenol is a key intermediate in industry for the synthesis of different classes of chemicals, such as pharmaceuticals. Unfortunately, the discovery of an efficient process to produce phenol remains an important challenge to solve in modern organic chemistry.
Basically, the direct introduction of a hydroxyl functionality into an aromatic C-H bond through chemical oxidation is challenging because of the notoriously low reactivity of aromatic C-H bonds (112 kcal·mol-1) and the low product selectivity caused by over-oxidation of phenols (see Figure 1 ).
The benchmark of the research is focused on first-row transition metal complexes as catalysts for aromatic oxygenation reactions using pyridylalkylamine ligands.1-2 Concretely, we focus into nickel due to it is an earth-abundant element, as well as cheaper and more benign than noble metals. Furthermore, a new strategy for trying to design and improve the efficiency of nickel complexes reported until date is focusing on the introduction of bulky substituents onto the pyridine rings of the ligands, as has been reported for some site-selective oxidation reactions.3-4
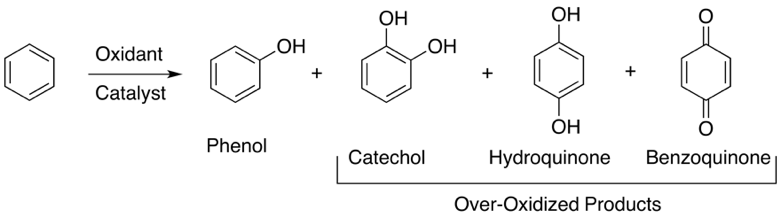 |
| Figure 1: Direct hydroxylation of benzene to phenol. Representation of the possible over-oxidized by-products. |
References:
[1] Morimoto, Y.; Bunno, S.; Fujieda, N.; Sugimoto, H.; Itoh, S., J. Am. Chem. Soc. 2015, 137 (18), 5867-5870.
[2] Tsuji, T.; Zaoputra, A. A.; Hitomi, Y.; Mieda, K.; Ogura, T.; Shiota, Y.; Yoshizawa, K.; Sato, H.; Kodera, M., Angew. Chem.-Int. Edit. 2017, 56 (27), 7779-7782.
[3] Cussó, O.; Cianfanelli, M.; Ribas, X.; Klein Gebbink, R. J.; Costas, M., J. Am. Chem. Soc. 2016, 138 (8), 2732-2738.
[4] Font, D.; Canta, M.; Milan, M.; Cussó, O.; Ribas, X.; Klein Gebbink, R. J.; Costas, M., Angew. Chem. Int. Ed. 2016, 55 (19), 5776-5779.